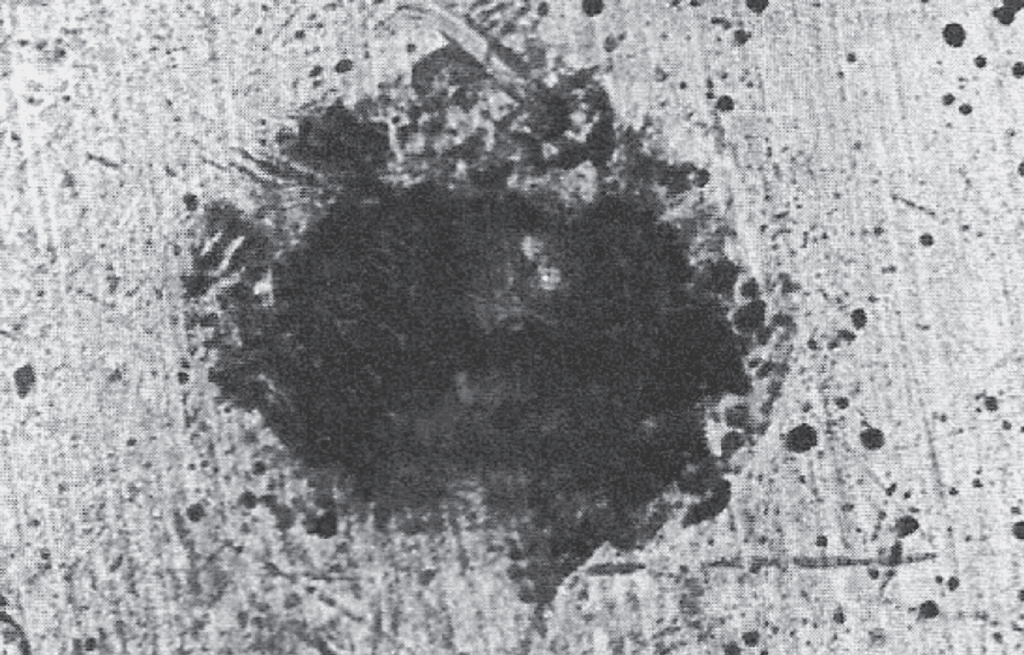
Silver surfaces react with sulfur and chlorine. Silver sulfide films, tarnish, are readily displaced on connector mating, but have been problematic in low force, non-wiping relay applications. Fretting displacement of silver tarnish does not result in fretting corrosion as described for tin fretting. Silver chloride is more tenacious, but less common as compared to silver tarnish.
Silver platings are generally applied in the range of 1.25 to 2.5 µm (50 to 100 microinches), and are soft compared to gold platings. The durability of silver finishes is lower than that of gold, but higher than that of tin.
Silver is recommended for power applications due to the high conductivity of silver and an increased resistance to arcing degradation compared to gold or tin finishes.