The majority of connector housings are manufactured from thermoplastic polymers due to their combination of electrical and mechanical properties and the manufacturing efficiencies of injection molding. This section will provide an overview of polymer materials and properties and a brief introduction to injection molding.
The word “polymer” describes the structure of polymers in that its meaning is “many mers” and the structure of a polymer is the repetition of a characteristic unit, the mer, many times, thousands of times. The properties of the polymer depend on the type of mer and the number of repetitions. The mers commonly used in engineering polymers are generally compounds among carbon and hydrogen, nitrogen, sulfur, chlorine and fluor.
The simplest carbon based compound is methane, CH4, a gas. The structure of methane is tetrahedral as shown in Figure 1.32 In contrast to metals where the outer electrons are free, as noted in Chapter I/1.4.2.1 Copper Alloy Metallurgy, the four outer electrons of carbon form shared directional bonds with four hydrogen atoms, each of which have one electron, to form methane gas. The electrons are shared between the carbon and hydrogen and are called covalent bonds. Covalent bonds are quite stable and methane is a stable molecule.
There are two variants of a carbon-hydrogen compound containing two carbons, ethane and ethylene. Ethane bonds use two electrons, the same as methane, as shown in Figure 1.33a. Ethylene used two electrons between carbon and hydrogen, but four electrons between the carbons, a so called double bond, as shown in Figure 1.33b. Ethylene is the simplest mer that can be polymerized and is the source of a family of polymers, polyethylenes. The polymerization reaction uses heat, pressure and catalysis to break the double bonds so that each carbon atom has a “dangling bond”, Figure 1.33c, which can, in turn combine with a “dangling bond” of an adjacent ethylene mer producing a chain of two ethylene molecules, Figure 1.33d. This process is called addition polymerization, mers are simply added on at each end of a polymer chain. A second polymerization process, condensation polymerization, will be discussed in a following section.
As noted the properties of the polymer vary with the number of mers. The number of mers is generally characterized in terms of the molecular weight of the polymer. For example, the molecular weight of the ethylene mer is:
As will be discussed later, polyethylene goes from a gas to a liquid to a grease to a wax, to a microcrystalline wax to a plastic as the molecular weight, the number of mers in the polyethylene chain increases. High Density High Molecular Weight (HDHMW) polyethylene has a molecular weight in the millions of ethylene mers. The structure and properties of a polymer chain depend on factors in addition to molecular weight.
Polymer Microstructure and Types
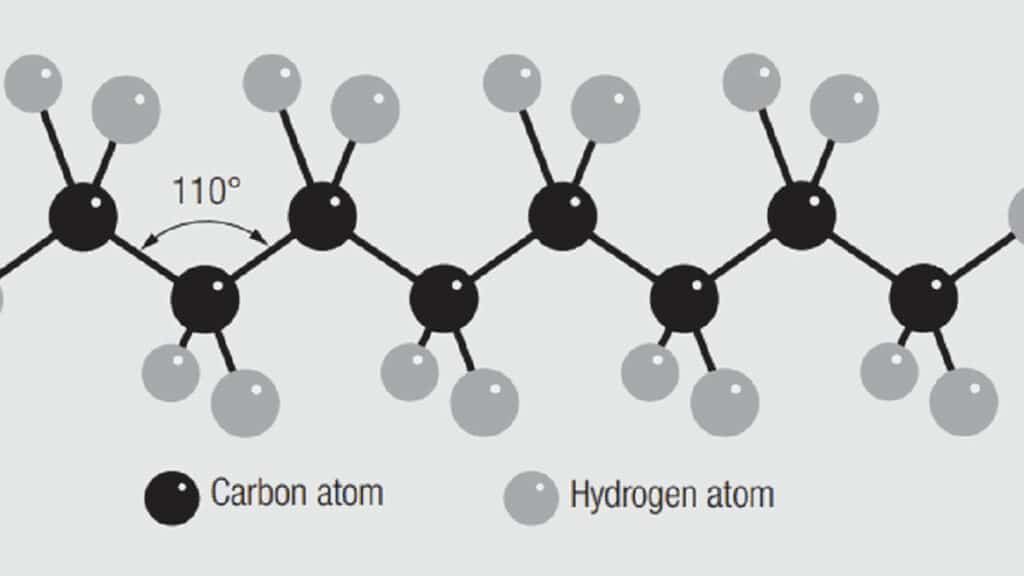
Consider now some other aspects of polymer bonding and how it affects the structure and properties of polymer chains. Two of the major aspects are bond angles and side groups. Figure 1.34 shows a schematic polyethylene chain. The carbon atoms are shown as black circles and are the backbone of the polymer chain. The hydrogen atoms, in grey, are the side group in polyethylene. There is a characteristic covalent bond angle, 110 degrees, between the carbon atoms in the backbone of a polyethylene chain. That angle depends on the bond energy between carbon and the side group and the size of the side group. For example other side groups in an ethylene type structure are methane, chlorine and fluorine which result in the polymers polypropylene, polyvinylchloride (PVC) and polytetrafluoroethylene (PTFE) respectively. Figure 1.35 shows the mers of these variants. Naturally the bond strengths, therefore the bond lengths, and bond angles of these polymers would be different from one another. Thus the interactions of the chains in the bulk polymer would be different resulting in the different properties.
As the length of the polymer chains increases, the directionality of the bonds tends to cause the chain to twist. The degree and form of twisting depends on the side groups on the chain and how they attach to the chain, randomly or at preferred sites, whichwould, in turn affect how the chains pack together. The reactivity of the side groups can also cause branching, an offshoot chain, or cross linking between chains resulting in three chain configurations, linear, branched and crosslinked as schematically illustrated in Figure 1.36.
These differences in chain interactions result in two fundamentally different microstructures, amorphous and crystalline polymers and two fundamentally different thermal characteristics, thermosetting and thermoplastic polymers. The former significantly affects the mechanical properties of a polymer and the latter the processing characteristics. Most of the engineering polymers used in connectors are crystalline or semi-crystalline thermoplastic materials: crystalline for the properties and thermoplastic for processing efficiency.
Polymer Structure and Mechanical Properties
The following discussion will be rather qualitative than quantitative to highlight the mechanisms of polymer deformation and how they vary with the polymer molecular weight and crystallinity. As noted previously, as the molecular weight of a polymer increases the length of the individual polymer chains increases which leads to two significant mechanisms of polymer strength, one physical and one chemical. First, as the length of the chains increases they are more likely to become entangled with one another. A common analogy being based on spaghetti; cut spaghetti reacts as individual strands while full length spaghetti reacts as a mass. Second, the increased chain length causes an increase in electrostatic bonding between the chains. Suffice it to say that there are several variations of dipolar bonding that can be active between polymer chains. Though the individual bond energies are small, the number of bonds between chains can be very large. In addition the electrostatic bonding tends to align the chains enhancing the opportunity for further bonding. This tendancy for alignment is the source of crystallinity in polymers. Figure 1.37a illustrates the random entanglements of an amorphous polymer, Figure 1.37b an intermediate stage of crystallinity and Figure 1.37c a highly crystalline polymer. The crystallinity of a polymer can be controlled by composition and processing. Note in Figure 1.37b that there are chains that are included in more than one crystallite cluster. These so called “tie molecules” in combination with the crystallites are an additional “entanglement” mechanism that is more stable than the physical entanglement previously described.
At low molecular weights, very short chains, the chains are free to slide over each other under an applied load. In the liquid and grease stages the chains flow freely and disentangle from each other. Eventually the increase in chain length promotes interchain bonding so that not all entanglements are disrupted, and the polymer becomes a wax. Increasing dipole bonding promotes increasing crystallinity leading to microcrystalline waxes and, eventually to rigid polymers, plastics. It should be noted that the dipole bond strength and frequency on the chain depend on the side groups in the chain so the tendency towards crystallinity is dependent on the side groups, the mers of the polymer. Crystalline polymers used in connectors include, polyamides (nylon), polyesters, and polyphenylenesulfide. Comments on these polymers will be provided later.
There is another class of polymers important to connector technology, Liquid Crystal Polymers (LCP). As the name indicates, LCP are crystalline in the liquid state consisting of rod like structures that readily self align into crystallites. At the temperatures where the LCP is liquid, thermal agitation limits the degree of crystallinity that is realized, but as the temperature decreases during the molding process a high degree of crystallinity is achieved.
The mechanical properties of many engineering polymers are further improved by the addition of glass fibers. The strengthening effect of glass fibers arises from chain bonding to the fibers. For example addition of glass fibers to the polyester PBT (polybutylene terephthalate) can double the tensile strength and increase the flexural modulus (analog to Elastic modulus in bending) by 250 percent.
A comparison of the mechanical properties of metals and polymers may be useful at this point. Metal strength data is generally provided via tensile stress. Polymer strength data may be obtained from either tensile or bending stress tests. The table in Figure 1.38 contains tensile data for copper30zinc brass in a temper commonly used in connectors and bending data for a commonly used PBT polyester polymer. The polymer data includes neat (unfilled) and 15 and 30 percent glass reinforced grades. Elastic modulus corresponds to flexural modulus and yield strength corresponds to flexural strength in the table.
There are several significant differences between the mechanical properties of metal and polymers in Figure 1.38: First, metals are much stiffer and stronger than polymers. For reference, the elastic moduli of copper alloys used in connectors fall in the range of 110 to 140 GPa (16 to 20 Mpsi) while for typical connector engineering polymers the corresponding range is 1 to 19 GPa ( 0.3 to 2.8 Mpsi); the corresponding comparison for tensile (flexural) strength are 230 to 760 MPa ( 35 to 110 Kpsi) for metals and 40 to 185 MPa (6 to 27 Kpsi) for polymers. Second, the effect of reinforcing glass fibers on polymer mechanical strength is significant. In the example polymer the flexural modulus and flexural strength are doubled with 15 percent glass, and nearly tripled with 30 percent glass. Similar improvements occur with other polymers. Third, it merits noting that the higher values of flexural modulus and strength are for Liquid Crystal Polymers and that LCP do not benefit from reinforcing fibers. A final, and significant difference between metals and polymers is their temperature and strain rate sensitivity. The mechanical properties of metals are essentially constant with respect to temperature and strain rate. Polymers, in contrast are sensitive to both. The mechanical properties of polymers are reduced as a function of increasing temperature and decreasing strain rate. A simplistic, but basically correct, explanation of these sensitivities revolves around the movement of polymer chains as a function of temperature and load. Increasing temperature increases the amount of thermal motion of the chains and facilitates their untangling and sliding over each other, thus reducing the strength of the material. Slow strain rates have a similar effect, the chains have more time to untangle and slide as the load slowly increases, viscoelasticity. Glass reinforced polymers are less sensitive to these temperature and strain rate effects due to the polymer bonding to the glass fibers restricting the chain motion. Metals are not immune to thermal effects as noted earlier with respect to stress relaxation and its effect on contact force. Polymers, however, show much larger effects, material flow, creep, rather than stress relaxation which is a localized atomic level rearrangement.